Drug administration withdraws medicines over cancer fears
by ,http://vietnamnews.vn/society/536420/drug-administration-withdraws-medicines-over-cancer-fears.html04 October 2019 Last updated at 17:54 PM
HÀ NỘI — The Drug Administration of Việt Nam, under the Ministry of Health, has decided to withdraw 11 drugs containing N-Nitrosodimethylamine (NDMA) beyond permitted levels that potentially cause cancer.
The administration said under current regulations, the NDMA limit must not exceed 0.32 parts per million (calculated on the maximum acceptable dose of NDMA as 96 nanograms per day).
Under a legal document signed by deputy head of the administration Đỗ Văn Đông, the 11 drugs come in the form of film-coated tablets, IV drugs and syrup.
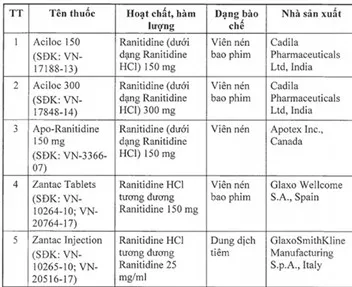
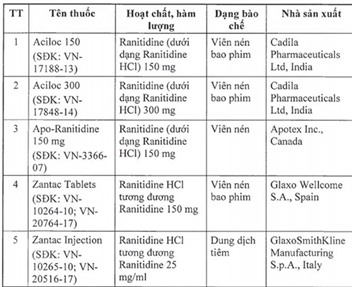
The drugs on the list are Aciloc 150; Aciloc 300; Apo-Ranitidine 150 mg; Zantac Tablets; Zantac Injection; Ratylno-150; Hyzan Tablet 150; Vesyca film Ranitidine 150 mg; Neoceptin R 150; Vesyca Film coated tablet 150 mg; Xanidine Tablet 150 mg; and Zantac Syrup 150 mg/10 ml.
All the drugs, which are used to treat stomach and intestinal ulcers, originated from India, Spain, Italy, Britain, Thailand, Canada and Malaysia.
The Singaporean Health Sciences Authority and the Swiss Agency for Therapeutic Products have also stopped the sale and supply of the drugs at clinics, hospitals and pharmacies.
The drug administration has ordered all companies that import the 11 drugs to publicise and withdraw them from circulation nationwide.
They have been given one month to report back on the amount of drugs they had imported and the amount they had managed to withdraw.
According to the Singaporean Health Sciences Authority, NDMA is a nitrosamine impurity. Nitrosamines are environmental contaminants and can be found in food or the environment in very minute amounts.
For example, NDMA can be found in processed food (pickled vegetables, salted fish, processed meat products such as bacon and sausages). Nitrosamine impurities have recently also been found to be formed unexpectedly during the manufacture of some medicines. Recalls have been undertaken worldwide for affected products found to contain these impurities above the acceptable levels. Studies have reported that exposure over a prolonged period of time to doses of nitrosamine impurities that are much higher than usual human exposure could cause cancer. — VNS





