Second COVID-19 vaccine to begin human testing in Vietnam
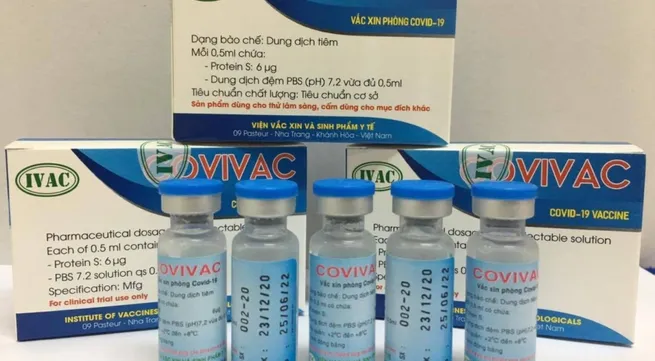
Covivac has proved to be safe and effective in experiment following pre-clinical studies in India, the United States and Vietnam, said Ivac director Duong Ngoc Thai.
The liquid vaccine, with or without adjuvants, is preservative-free, using the Newcastle disease virus (NDV) vector vaccines expressing the spike protein of SARS-CoV-2.
Ivac got down to work on the project in May 2020 and after seven months it had successfully produced three batches on a large scale numbering between 50,000 – 100,000 doses each.
The vaccine has triggered a promising immune response and appeared safe in animals in pre-clinical experiments both locally and overseas, said Dr. Thai.
Covivac can protect those injected against variant strains of SARS-CoV-2 from the United Kingdom and South Africa, said Dr. Thai, adding scientists are studying the efficacy of the vaccine against other strains.
The human clinical trial of the vaccine is to begin in March 2021 and is expected to be complete in October 2021.
The first phase of the testing campaign will be carried out at Hanoi Medical University, and the second phase at a medical centre in Vu Thu District, Thai Binh province.
Vietnam is entering the second phase of its human clinical trial of the first locally-produced vaccine Nano Covax. The vaccine is said to generate antibodies that help protect volunteers against the UK variant strain.





